Watch this beautiful video and forget about analysis and visualisation of high dimensional data. How could you represent the "spirit" of Cha Dao on a graph?
"The culture of drinking tea springs from the heart and becomes part of life."
That lab experiment is going on all the time in our brains. It's the way we function. We can't help it. But, what is in our brains(thoughts) may not necessarily be true or factual. To take a position one way or another is not the way of the Tao, is it?tingjunkie wrote:What it all boils down to for me is this...
I can taste the difference, smell the difference, and feel the difference in my mouth. Notice that sentence was 100% personal to me. When I go to drink tea, there is no lab equipment in my mouth, or guys with lab coats and clipboards. So why would anyone need scientific tests to prove Yixing clay's effect on anything? Like Bob Marley said, "who feels it, knows it." You either feel it or you don't, and no lab tests are required once you do!
If you are the type that needs scientific verification, you should probably stick to a gaiwan, and not drive yourself crazy. Happy tea drinking to you, and please let us know if you find any new exceptional teapot clays from elsewhere. I'm not stuck on tradition.
Well in that case... There is no tea. There are no pots.Tead Off wrote:To take a position one way or another is not the way of the Tao, is it?In other words, there is only the personal which is neither true or false.
Let's not put words in my mouth. Just tea, please.tingjunkie wrote:Well in that case... There is no tea. There are no pots.Tead Off wrote:To take a position one way or another is not the way of the Tao, is it?In other words, there is only the personal which is neither true or false.
I think if anyone had achieved true detachment, they wouldn't be found here on the teaware forum. We're the biggest materialists in the tea world!
I did get a copy of this, and there is some interesting stuff in there. It's short, and has a lot of charts / graphs / etc. of mineral distribution. There's a little bit of "rah rah rah" in it, but there is some actual information about what makes zisha distinctive, if not unique. I will re-post some of it because I know you nerds are interested.battra wrote: Anyway, I did find an article:
An analysis of the chemical composition, performance and structure of China Yixing Zisha pottery from 1573 A.D. to 1911 A.D.

The article does also seem to confirm that the composition of the clay seems to have changed a bit over time:Fig. 1. Samples of the Zisha pottery shards of different periods unearthed in the Shushan Mountain kiln sites of Yixing, China, Note: the background grid 10 mm x 10 mm.
This section does allude to some of what we've been talking about:Generally speaking, the Fe2O3 content in these Zisha samples increases over time, ranging from 4.72% to 7.36% while the ratio of SiO2/Al2O3 contained, decreases first and then increases, that is, it decreases gradually from the late Ming period to the middle Qing period and then increases greatly in the late Qing period.
[...]
The ratios of SiO2/Al2O3 and RxOy/SiO2 of the samples from the late Qing Dynasty are close to those from the late Ming Dynasty, which are near the upper left-hand part of Fig. 5. That is, the ratio of SiO2/Al2O3 is relatively high while the ratio of RxOy/SiO2 low.
When using Yixing Zisha tea pots, people realize that they have many excellent utilitarian performances such as slow heat transfer, good insulation, high intensity, delicate appearance and good tea flavor, which are closely related to the selection and processing of its unique traditional raw materials and its firing techniques, etc.
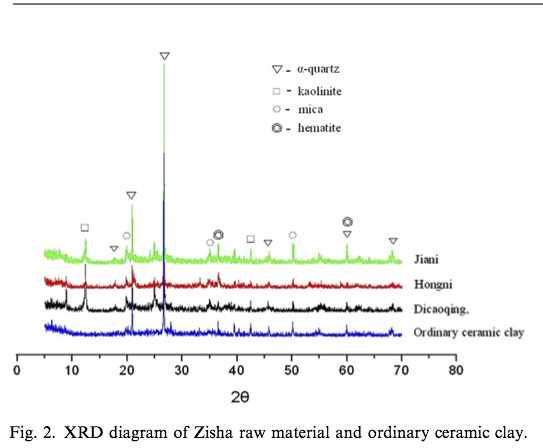
First, the selection of raw materials is unique. Fig. 2 is the XRD diagram of some kinds of common Zisha raw materials (Dicaoqing, Jiani and Hongni) and ordinary local ceramic clay. The mineral composition of the Zisha raw materials consists of mica, kaolinite, a-quartz, and some hematite. Compared with the ordinary ceramic clay, the content of kaolinite in Zisha raw materials is relatively high. The kaolinite crystals in small strips endow Zisha clay with good plasticity while the high content of quartz can reduce the drying shrinkage and firing shrinkage. These are of great significance for the improvement of the strength of the bodies in the process of making Zisha pottery. In addition, the raw material of Yixing Zisha has high Fe2O3 content (~7%) which is equally distributed [2]. FeO decomposed from Fe2O3 during high temperature stage of firing process forms low melting point silicate melts combined with SiO2. It is helpful to promote the sintering degree of body and increase the mechanical strength of products.
Second, the traditional technological process of Yixing Zisha raw materials is much more complicated than that of ordinary potteries and needs more than one processing before it can be used. For example, usually, the freshly exploited Zisha raw materials are as hard as stones which need a few months’ natural weathering before they can be ground into powder.
[talking about the construction strategy]
The continuous beating can press inward on the crude particles on the
surface of the body to make it flat and smooth, endowing Yixing Zisha pottery with unique gradient distribution of pore structure (Fig. 4). It can be found from Fig. 4 that the pores in the outer layer of the Zisha samples are small in size and round in shape, distributed equally in the body whose porosity ranges from 5% to 9%. From the middle layer on, the size of the pores begin to increase gradually and these pores in the shape of small lines scattered in the body with porosity ranging from 9–14%. The chain-like pores in the inner layer are without regular shape, scattered, and inter-crossing in the inner surface of the body, with porosity ranging from 15% to 17%. Therefore, from the outer to the inner layer of the Yixing Zisha pottery, the porosity increases gradually, forming a multi- pore reticulation in vertical gradient distribution.

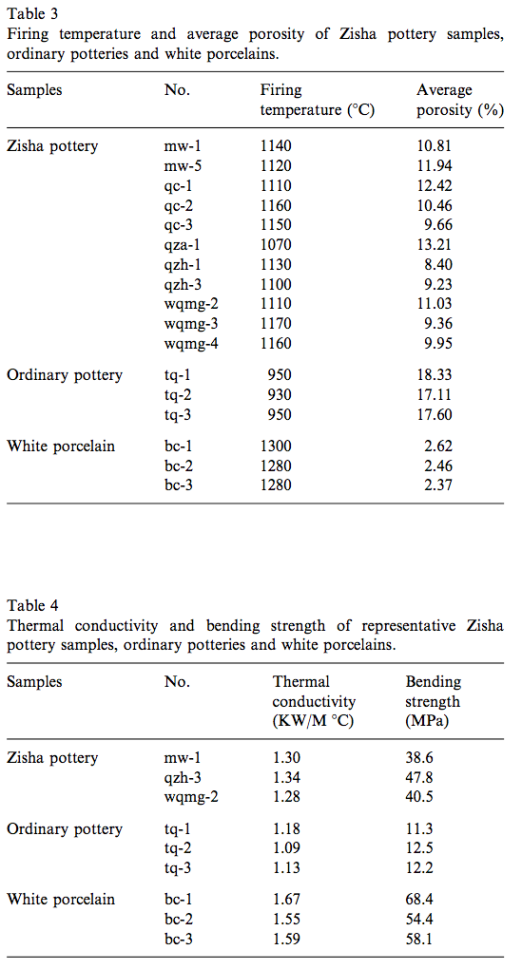
In addition, the firing temperature of Yixing Zisha pottery is higher than that of ordinary Chinese potteries (~800 1C [12]), reaching around 1150 °C (Table 3). Fig. 3 is the XRD diagram of the Zisha samples of two representative periods, namely the late Ming Dynasty (mw-5) and the mid-Qing Dynasty (qzh-1). From Figs. 2 and 3 it can be found that from raw materials to the fired objects, their phase composition has undergone great changes. This is because the kaolinite in Zisha raw materials, after being dehydrated at 450–650 °C, has been decomposed to be metakaolinite, which, with the further rise of temperature, is transformed into Al–Si spinel. Some of Al–Si spinel, at the temperature around 1050 °C, is transformed into primary mullite and formless quartz.
When it reaches 1150 °C, some of the mica in the raw material is transformed into glass phase [13]. This makes the phase composition of Yixing Zisha pottery different from that of the ordinary Chinese pottery fired at a lower temperature. The former with low porosity (Table 3) and more compact body can ensure itself with relatively high mechanical strength.
All quotes and images from:The unique inner pore structure and phase composition of Yixing Zisha pottery has endowed it with excellent performance as tea sets.
[...]
The thermal conductivity of the white porcelain is the largest while that of the ordinary pottery the smallest and that of the Zisha pot lies right in between. In ceramic material, the pore is a kind of dispersion phase which hinders the heat transfer and usually can reduce the thermal conductivity of the matrix. The ordinary pottery, due to its high porosity reaching around 18%, has the lowest thermal conductivity and good insulation but loose texture, low mechanical strength and crude appearance. Therefore, it is unsuitable for tea sets. As to white porcelain pot, although with high mechanical strength and delicate appearance, its high thermal conductivity and poor insulation makes it difficult for the extracts of the tea to dissolve fully, affecting, to some extent, the traditional Chinese tea-sipping effect. Yixing Zisha pottery, however, has found a good equilibrium point.
There's more in the article that's not quoted. However, I wondered the same thing - I didn't see an explanation at how they arrived at the firing temperature so precisely.Drax wrote:Thanks for sharing that article, wyardley!
Sort of a tangent, but on their table 3, did they mention how they know what the firing temps were? Those seem like pretty precise values, so I'm curious how they figured it out (maybe it has something to do with the ratios of isoforms of certain crystals present...?)
Wonderful! Thanks Mr. M in advance!!MarshalN wrote:I'd imagine they can find out the temps based on what kind of chemicals exist in what form in the clay?
Incidentally, I had someone do some spectral analysis on two pots of mine. Results should be coming in this week.